VETERINARSKI ARHIV 68 (3), 109-119, 1998
ISSN 0372-5480
Printed in Croatia
Selection of immature bovine oocytes as the preliminary phase of in vitro fertilisation
Zdenko Makek1*, Iva Getz1, Marijan Cergolj1,
Miroslav Herak1, Antun Tomaškovic1,
Korana Stipetic1,
Tomislav Dobranic1, and Velimir Sušic2
1Department of Reproduction and Clinic of Obstetrics, Faculty of Veterinary
Medicine,
University of Zagreb, Zagreb, Croatia
2Department of Animal Husbandry,, Faculty of Veterinary Medicine,
University
of Zagreb, Zagreb, Croatia
* Contact address:
Prof. Dr. Zdenko Makek,
Department of Reproduction and Clinic of Obstetrics, Faculty of Veterinary Medicine, University of Zagreb, 10000 Zagreb, Heinzelova 55, Croatia,
Phone: 385 1 23 90 321, Fax: 385 1 214 697
MAKEK, Z., I. GETZ, M. CERGOLJ, M. HERAK, A. TOMAŠKOVIC, K. STIPETIC, T. DOBRANIC, V. SUŠIC: Selection of immature bovine oocytes as the preliminary phase of in vitro fertilisation. Vet. arhiv 68, 109-119, 1998.
ABSTRACT
Ten Holstein-breed cows, 3 to 7 years old, with an unknown reproductive history were selected from a feedlot and underwent gynaecological examination in order to determine the stage of the oestrus cycle and to choose possible donors of immature oocytes for in vitro maturation and fertilisation. Two cows, in which ovarian atrophy and cysts were diagnosed, were excluded from the research. After the slaughter of the remaining 8 cows, immature oocytes were aspirated from follicels sized more than 2 mm. Phase of the oestrus cycle was determined for each individual cow, based on vaginal, rectal and ultrasound examinations and on a morphological evaluation of pairs of ovaries and corpus luteum mass. Follicles were classified by size into four categories. Follicles determined by rectal and ultrasound examinations, were compared with those identified by macroscopic assessment. Number and category of aspirated oocytes were sorted according to the phase of the oestrus cycle. Immature bovine oocytes were morphologically evaluated under a stereomicroscope (magnification ×50, ×100, ×210). They were then classified into three categories: (1) oocytes surrounded by compact multi-layers of follicle cells and even, granulated ooplasm; (2) oocytes with partial cumulus present, or enclosed by 1-2 layers of compact cumulus cells and granules clumped or unevenly distributed in ooplasm, and (3) oocytes partially surrounded with less compact expanded cellular investment and "nude" oocytes with degenerated, vacuolated or fragmented ooplasm. The authors concluded that morphological evaluation of the layer of follicular cells surrounding immature oocytes and their ooplasm could be used as a criterion for selection of immature oocytes suitable for in vitro maturation and fertilisation. Additionally, ultrasound examination of the ovaries prior to aspiration is a simple and non-invasive method for the effective selection of potential donors of immature bovine oocytes.
Key words: cow, follicle, immature oocyte, aspiration
Introduction
The use of in vitro techniques has become a routine procedure in many laboratories for the large-scale production of embryos that are used in fundamental gametes physiology investigations, as well as in reproductive biotechnology such as twining, cloning, pre-implantation diagnosis of embryos (sexing), transgenic animal production, studies on embryonic mortality, the use of oocytes from calves and, finally, for the more efficient use of semen (GREVE at al., 1993). Advanced embryo technologies are also used for the large-scale production of embryos in commercial cattle breeding (GORDON and LU, 1990). Bovine oocytes (cumulus oocytes complexes, COCs) can be aspirated either from the living animal, by a transvaginal ultrasound puncture from super-ovulated or normally cycling cows (PIETERSE et al., 1988), or from ovaries collected at the slaughterhouse. The ovaries from culled animals provide an almost inexhaustible source of follicular oocytes, and they are generally collected without taking into account the follicle-oocyte relationship. Ultrasound may be applied to examine at which stage of the cycle a maximum number of follicles is present for aspiration, and when follicles are in the growth or static phases (GREVE et al. 1993). Immature oocytes aspirated from follicles larger than 2 mm in diameter with homogenous ooplasm, and surrounded by a tight and complete multi-layered cumulus investment, are chosen for the in vitro maturation (IVM) procedure. Anticipation of their maturational and development potential in order to establish the difference between the "losers" and the "winners" among oocytes has great importance for the success of in vitro fertilisation (IVF) and culture (IVC) of bovine embryos up to the blastocyst stage (SHAMSUDDIN et al., 1996).
Visual assessment based on the morphological characteristics of the follicle cell investment surrounding immature bovine oocytes and its ooplasm can be used to select oocytes most capable of embryonic development following maturation and fertilisation in vitro (MADISON et al., 1992). Therefore we carried out an investigation, for the first time in the Republic of Croatia, into the selection of immature bovine oocytes for in vitro maturation, with the aim of establishing an in vitro procedure in Croatian veterinary medicine.
Materials and methods
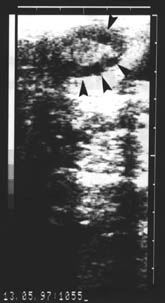
The study was performed out on ten Holstein cows, of unknown reproduction history, 3 to 7 years old, from the clinical depot PIK Vrbovec d.d. On the afternoon before slaughter the cows were thoroughly examined gynaecologically - vaginally, by rectal palpation, and then by ultrasound using an Aloca Echo Camera SSD-210 DX II device with the transrectal linear 5 MHz transducer. The echograms of each ovary were printed out on a Mitsubishi Video Copy AP-8600 printer, Japan (Fig. 1). The next morning the cows were slaughtered and their ovaries were removed within a maximum period of 30 minutes after slaughter. The ovaries were transported to a laboratory in a thermos of Ringer solution and antibiotic addition (100 I.U. penicillin and 100 µg streptomycin/ml) at temperature of 30 oC. After arrival at the laboratory the ovaries were washed in the Ringer solution, warmed to a temperature of 37 oC. All the forms on the ovaries were macroscopically described and the results compared with the rectal and ultrasound finds of each cow determined on the previous day (Fig. 2). The contents of follicles (diameter more than2 mm) visible at the ovarian surface were aspirated with a 5 ml syringe and an 18-gauge needle (outer diameter 1.2 mm) and placed in Ringer/antibiotic solution, warmed to 37 oC. Follicular contents were viewed under stereomicroscope, magnification ×15, ×100, ×210.

Fig. 1. Echogram of the cow ovary in follicular phase. Arrows mark follicles.
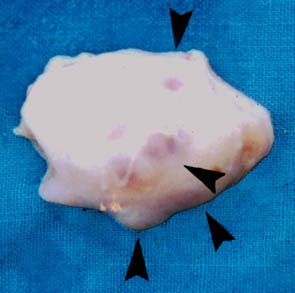
Fig. 2. Macroscopical image of the cow ovary shown in Fig. 1, 24 hours following the ultrasound examination. Arrows mark follicles.
The following was determined:
Oestrus cycle phase based on the vaginal, rectal and ultrasound findings and morphological evaluation of the ovaries and mass of the corpora lutea. The pairs of ovaries were classified in 3 oestrus cycle phases as described by LEIBFRIED and FIRST (1979):
Early luteal: ovaries showed evidence of a recent ovulation (corpus haemorrhagicum) or a developing corpus luteum (CL) of mass lesser than 2 grams (measured by analytic scale). The corpus albicantium of the previous oestrus cycle could be identified.
Luteal: CL of mass more than 2 grams and the uterine horns showed no pregnancy signs.
Follicular: CL in the regression weighing more than 3 grams, yellow and firm in texture.
Follicles classified according to their surface diameter: follicles on each ovary were counted and their outer diameter was measured. According to their size they were classified in 4 groups, lesser than 3 mm, 3 to 6, 6 to 10 and more than 10 mm.
Number of follicles determined by rectal and ultrasound examinations and macroscopical evaluation of the ovaries and follicles after slaughter were classified according to size.
Aspiration success and number of the aspirated oocytes of each oestrus cycle phase.
Morphological evaluation of oocytes. These were classified according to the appearance of cumulus cells and ooplasm, in 3 categories:
(1) oocytes completely surrounded by compact follicular cell layers (more than 3) and with an even granulated ooplasm that completely filled the zona pellucida;
(2) oocytes partially surrounded with the compact cumulus cell layer or with lesser than 3 cell layers of granulated ooplasm, with granules clumped or unevenly distributed in ooplasm; and
(3) cells incompletely surrounded with less compact cumulus cells with weakened internal connections and "nude" oocytes with degenerated ooplasm with vacuoles, fragmented, or with the remains of the ooplasm that did not completely fill the zona pellucida.
The number and category of aspirated oocytes were classified according to the oestrus cycle phase.
Results
The oestrus cycle phase was determined as the possible maturation ability indicator of the oocytes aspirated in vitro from the follicles with diameter more than 2 mm. The oestrus and follicles before ovulation were determined in 10 cows, and corpora lutea in 5 cows, by gynaecological examination (vaginal, rectal and ultrasound). In one cow the fresh corpus luteum (corpus haemorrhagicum) was determined postmortally, and not by rectal or ultrasound examination. Two animals were excluded from further research: in one the ovarial dystrophy was determined postmortally, and in the other a 3.9×3.7 cyst.
The following results concern the ovaries of the remaining 8 cows.
It was determined by the above explained morphological postmortal evaluation that 2 cows were in the follicular phase, 3 in the luteal, and 3 in the early, luteal oestrus cycle phase.
Classification of the follicles in groups according to their size, measuring the surface diameter is shown in Table 1 in relation to the oestrus cycle phase.
Table 1. Classification of follicles according to their size in relation
to the oestrus cycle phase
|
Follicle size |
Number of follicles in the different phase of the oestrus cycle (n) |
Total number |
||
|
Follicular phase |
Early luteal phase |
Luteal phase |
||
|
lesser than 3 |
38 (66.7 %) |
39 (66.1 %) |
22 (53.7 %) |
99 (63.1 %) |
|
3-6 |
14 (24.6 %) |
14 (23.7 %) |
11 (26.8 %) |
39 (24.8 %) |
|
6-10 |
2 (3.5 %) |
4 (6.8 %) |
5 (12.2 %) |
11 (7.0 %) |
|
more than 10 |
3 (5.2 %) |
2 (3.4 %) |
3 (7.3 %) |
8 (5.1 %) |
|
57 (100 %) |
59 (100 %) |
41 (100 %) |
157 (100 %) |
|
The number of follicle classified in groups according to their size, determined
by rectal and ultrasound examinations, and later by postmortal macroscopical
evaluation of the ovaries after slaughter, is shown in Fig. 3.
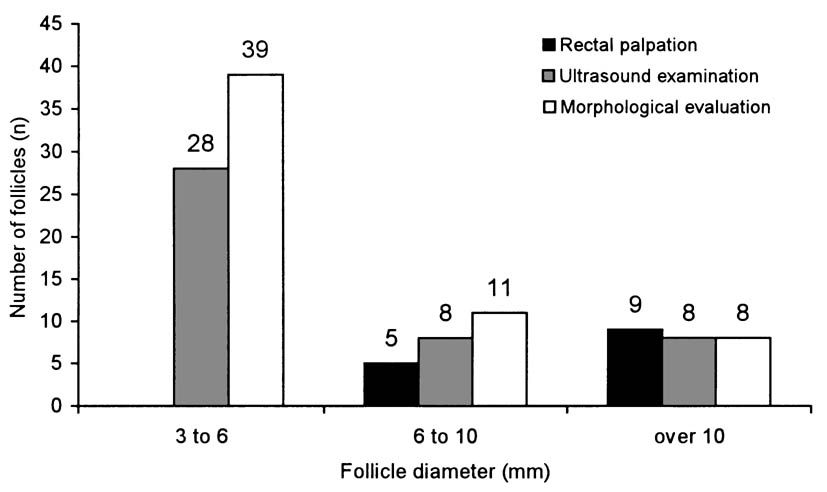
Fig. 3. Number of follicles in ovaries of the examined cows classified into size categories determined by rectal palpation, ultrasound examination and postmortal morphological evaluation
Aspiration success and the number of aspirated oocytes, according to the oestrus cycle phase, is shown in Fig. 4. A total of 157 follicles was determined, and 52 oocytes were aspirated out of the follicles of 8 cows. Two cows were in follicular phase, three in early luteal and three in luteal phase of the oestrus cycle.
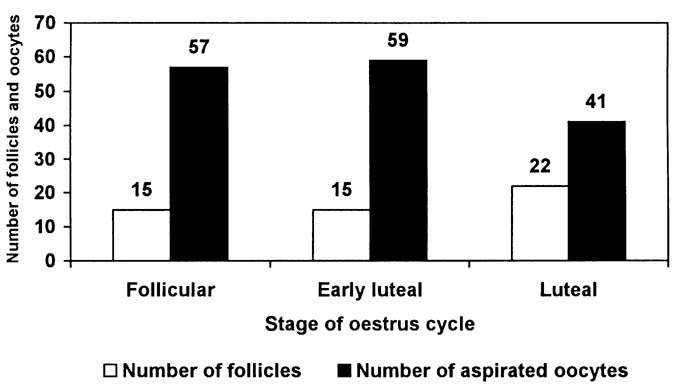
Fig. 4. Number of follicles in ovaries of the examined cows and aspirated oocytes classified according to the phase of oestrus cycle
Results of the morphological evaluation and the oocyte classification in the oestrus cycle phase are shown in Table 2.
Table 2. Oocytes classification according to cumulus cells and ooplasm
appearance in the different oestrus cycle phases
|
Oocyte |
Number of oocytes in the different phase of the oestrus cycle |
Total |
||
|
Follicular phase |
Early luteal phase |
Luteal phase |
||
|
(1) |
8 (53.3 %) |
5 (33.3 %) |
11 (50.0 %) |
24 (46.2 %) |
|
(2) |
6 (40.0 %) |
6 (40.0 %) |
8 (36.4 %) |
20 (38.5 %) |
|
(3) |
1 (6.7 %) |
4 (26.7 %) |
3 (13.6 %) |
8 (15.4 %) |
|
Total number |
15 (100 %) |
15 (100 %) |
22 (100 %) |
52 (100 %) |
*see "Materials and methods" chapter
First category oocytes (Fig. 5) are completely surrounded by several follicular
cell layers and evenly granulated ooplasm. They were present in the follicular
phase in 53.3%, in the luteal phase 50% and in the early luteal phase in
33.3%. Second category oocytes partialy surrounded by less then 3 layers
of cumulus cells and unevenly granulated ooplasm (Fig. 6) were present
alike in all oestrus cycle phases. Third category oocytes, unsuitable for
in vitro maturation, were present in the follicular phase in 6.7%, in the
luteal 13.6%, and in the early luteal phase 26.7%.
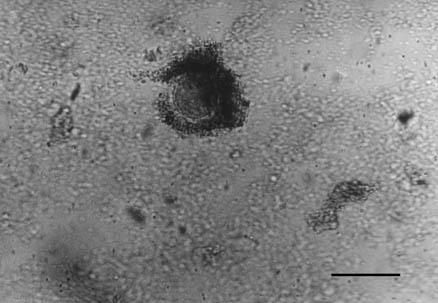
Fig. 5. First category of cow oocyte. Bar = 200 µm, × 210.
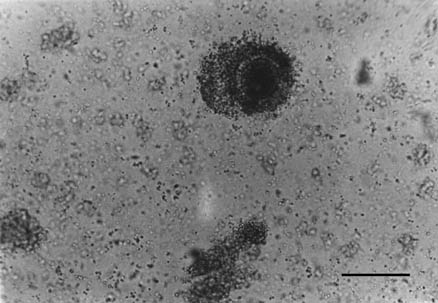
Fig. 6. Second category of cow oocyte. Bar = 200 µm, × 210.
Discussion
During fetal life, a pool of primordial follicles was established in the bovine ovary. Each of these follicles consists of an oocyte arrested in prophase I, or meiosis, and a single layer of flattened granulosa cells (FORTUNE, 1994). In each oestrus cycle, two or three follicular "waves" occur, in which 3-6 follicles simultaneously began to grow larger than 5 mm (SIROIS and FORTUNE, 1988). One of these follicles became dominant and continued its growth, whilst the other, subordinate, follicles regress. If the follicle is dominant at the time of luteal regression, it will become the ovulatory follicle, and will release a mature oocyte (Van SOOM and de KRUIF, 1996). It is, therefore, highly probable that the oocytes derived from follicles in the growing phase have a higher developmental capacity following IVM/IVF: it is related to the actual stage of the oestrus cycle at the time of oocyte collection, as stated by MACHATKOVÁ et al. (1996). They obtained the best blastocyst yields from oocytes isolated in the oestrus cycle days 14 to 16. THONON et al. (1994) gained a maximum number of transferable embryos following IVM/IVF/IVC from cows in the luteal phase of the cycle. Our results disagree with the cited data, since we collected the maximum number of 1st category oocytes (53.3%) from cows in follicular phase of the oestrus cycle. On the contrary, ARIOTTO et al. (1996) found that there is no difference in the developmental competence of oocytes between the three phases of the oestrus cycle.
With respect to size of the follicle from which the oocyte is recovered, LEIBFRIED-RUTLEDGE et al. (1989) did not establish that it influences the ability of the bovine oocyte to undergo nuclear maturation in vitro. LUCAS-HAHN et al. (1992) concluded that blastocysts from follicles diameter-ranging from more than 2 to 8 mm in size have a similar development potential. Oocytes from 1 to 2 mm follicles have a significantly lower competence to undergo IVM/IVF, and under experimental conditions completely lack the capability to cleave beyond the 8-cell stage. ECTORS et al. (1995) showed that this improved developmental capacity of oocytes from larger follicles (more than 6 mm) was not due to a better nuclear maturation, but presumably to a better cytoplasmic maturation. Such large follicles are probably among those competing for dominance. ARIOTTO et al. (1996) stated that development to the morula and blastocyst stages increases with increasing follicular diameter, also as the diameter of the oocyte increases. In our investigation the highest rate of follicles smaller than 3 mm was observed in follicular (66.7%) and early luteal phase (66.1%), whereas the highest rate of follicle size categories 3 to 6 mm (26.8%) and 6 to 10 mm (12.2%) was observed in the luteal phase; those size classes in which better developmental potential for IVM/IVF of oocytes could be expected.
Monitoring of follicular growth patterns by ultrasound and aspiration of immature oocytes by the transvaginal technique, as stated PIETERSE et al. (1988), may provide us with the ability to examine the development capacity in vitro of oocytes collected at known phases of follicular growth and stages of degeneration. GREVE et al. (1993) consider that ultrasound, followed by aspiration and maturation in vitro, can be used to determine the size a follicle must reach before the oocyte has completed growth and is capable of maturation in vitro. GANDOLFI et al. (1997) studied whether the developmental potential of bovine COCs, selected on the basis of widely accepted morphological parameters, can be related to the morphology of their originating ovary, providing a more simple and objective selection criterion. Their results have been positive, since ovarian morphology is easy to evaluate and does not require any extra work when isolating COCs for in vitro maturation.
In our trial, we collected 52 oocytes by aspiration of 157 follicles from 16 ovaries (8 cows). THONON et al. (1994) yielded an average of 24 oocytes per cow, with great individual differences (ranging from 0 to 100), but approximately 50% were eliminated from further procedures, based on classical morphological criteria of selection. ARIOTTO et al. (1996) recovered approximately 25 oocytes per ovary by first aspirating surface visible follicles, and subsequently mincing ovaries release oocytes from cortical follicles. Retrieving oocytes from both follicular populations approximately doubled the total yield of oocytes, compared with aspiration alone. Inadequate success of aspiration in our investigation was probably due to the long interval and manipulation of ovaries from the collection at the abattoir until the aspiration of oocytes.
Conclusion
The presented data indicate that ultrasound may be applied to examine at which stage of the cycle the maximum number of follicles suitable for aspiration is present on the ovaries, which proves that ultrasound examination of the ovaries is a simple and non-invasive method for effective selection of potential donors of immature bovine oocytes. In addition, morphological evaluation and classification of oocytes, based on visual assessment of the follicular cell mass and their ooplasm, can be used to select immature oocytes most capable of maturation, fertilisation and cleavage in vitro.
Acknowledgements
This research was supported by a research grant (No 053040)
received from the Ministry of Science and Technology of the Republic of
Croatia.
References
ARIOTTO T., J. L. SCHWARTZ, N. L. FIRST, M. L. LEIBFRIED-RUTHLEDGE (1996): Aspects of follicle and oocytes stage that affect in vitro maturation and development of bovine oocytes. Theriogen. 45, 943-956.
ECTORS, F. J., L. KOULISCHER, M. JAMAR, C. HERENS, A. VERLOES, B. REMY, J. F. BECKERS (1995): Cytogenetic study of bovine oocytes matured in vitro. Theriogen. 44, 445-450.
FORTUNE, J. E. (1994): Ovarian follicular growth and developmental in mammals. Biol. Reprod. 50, 225-232.
GANDOLFI, F., A. M. LUCIANO, S. MOLDINA, A. PONZINI, P. POCAR, D. T. ARMSTRONG, A. LAURIA (1997): The in vitro developmental competence of bovine oocytes can be related to the morphology of the ovary. Theriogen. 48, 1153-1160.
GORDON, I., K. H. LU (1990): Production of embryos in vitro and its impact on livestock production. Theriogen. 33, 77-87.
GREVE, T., V. MADISON, B. AVERY, H. CALLESEN, P. HYTTEL (1993): In vitro production of bovine embryos: a progress report and the consequences on the genetic upgrading of cattle populations. Anim. Reprod. Sci. 33, 51-69.
LEIBFRIED-RUTLEDGE, M. L., N. L. FIRST (1979): Characterization of bovine follicular oocytes and their ability to mature in vitro. J. Anim. Sci. 48, 78-86.
LEIBFRIED-RUTLEDGE, M. L., E. S. CRITSER, J. J. PARRISH, N. L. FIRST (1989): In vitro maturation and fertilization of bovine oocytes. Theriogen. 31, 61-74.
LUCAS-HAHN, A., A. PAVLOK, H. NIEMANN (1992): Competence of bovine oocytes derived frim different size follicles. Reprod. Dom. Anim. 27, 60-61.
MACHATKOVÁ, M., E. JOKEŠOVÁ, J. PETELIKOVÁ, V. DVORÁCEK (1996): Developmental competence of bovine embryos derived from oocytes collected at various stages of the oestrus cycle. Theriogen. 45, 801-810.
MADISON, V., B. AVERY, T. GREVE (1992): Selection of immature bovine oocyes for developmental potential in vitro. Anim. Reproduc. Sci. 27, 1-11.
PIETERSE, M. C., K. A. KAPPEN, TH. A. M. KRUIP, M. A. M. TAVERNE (1988): Aspiration of bovine oocytes during transvaginal ultrasound scanning of the ovaries. Theriogen. 30, 751-762.
SHAMSUDDIN, M., K. NIWA, B. LARSSON, H. RODRIGUEZ-MARTINEZ (1996): In vitro maturation and fertilization of bovine oocytes. Reprod. Dom. Anim. 31, 13-22.
SIROIS, J., J. E. FORTUNE (1988): Ovarian follicular dynamics during the oestrus cycle in heifers monitored by real time ultrasonography. Bio. Reprod. 39, 308-317.
THONON, F., F. J. ECTORS, A. DELVAL, H. LENS, K. TOUATI, J. F. BECKERS, F. ECTORS (1994): Le point sur la reproduction d,embryos bovins in vitro: limitations et perspectives de la recherche. Ann. Med. Vet. 138, 33-40.
Van SOOM, A., A. de KRUIF (1996): Oocyte maturation, sperm capacitation and pre-implantation development in the bovine: implications for in vitro production of embryos. Reprod. Dom. Anim. 31, 687-701.
Received: 25 April 1998
Accepted: 23 May 1998
MAKEK, Z., I. GETZ, M. CERGOLJ, M. HERAK, A. TOMAŠKOVIC, K. STIPETIC, T. DOBRANIC, V. SUŠIC: Odabir nezrelih govedih jajasaca kao pripremna faza u postupku oplodnje in vitro. Vet. arhiv 68, 109-119, 1998.
SAZETAK
Ginekološki (vaginalno, rektalno i ultrazvucno) pregledano je 10 crno-šarih krava starosti od 3 do 7 godina iz klaonickog depoa, nepoznate reprodukcijske anamneze, da bi se za svaku kravu odredili faza spolnog ciklusa i odabrale moguce davateljice nezrelih jajasaca za postupak dozrijevanja i oplodnje in vitro. Dvije zivotinje, u kojih su dijagnosticirane atrofija jajnika i ciste, iskljucene su iz daljnjeg postupka. Nakon klanja preostalih 8 krava, aspirirana su nezrela jajasca iz folikula promjera veceg od 2 mm. Za sve krave utvrdeni su: faza spolnog ciklusa na osnovu vaginalnog, rektalnog i ultrazvucnog nalaza te morfološke ocjene jajnika i mase zutog tijela. Folikuli su svrstani na osnovu promjera u cetiri razreda, te usporedeni oni koji su utvrdeni rektalnom i ultrazvucnom pretragom s makroskopskim pregledom nakon klanja. Nezrela jajasca morfološki su ocijenjena i klasificirana u tri kategorije: (1) jajasca potpuno okruzena kompaktnim slojem folikularnih stanica i fino granuliranom ooplazmom; (2) jajasca djelomicno okruzena kompaktnim slojem stanica kumulusa ili s manje od 3 sloja stanica i s neravnomjerno granuliranom ooplazmom te (3) jajasca nepotpuno okruzena stanicama kumulusa s oslabljenim medustanicnim vezama te "gola" jajasca s degeneriranom ooplazmom. Broj i kategorija aspiriranih jajasaca razvrstana je po pojedinim fazama spolnog ciklusa. Kao jedan od kriterija pri odabiru jajasaca najpogodnijih za postupak in vitro dozrijevanja i oplodnje moze posluziti morfološka procjena sloja folikularnih stanica koji okruzuje nezrelo govede jajasce i njezinu ooplazmu.
Kljucne rijeci: krava, folikul, nezrelo jajasce, aspiracija